| ANAESTHESIA PEARLS |
- How Safe is to Give Neuraxial Blocks in Patients with Thrombocytopenia
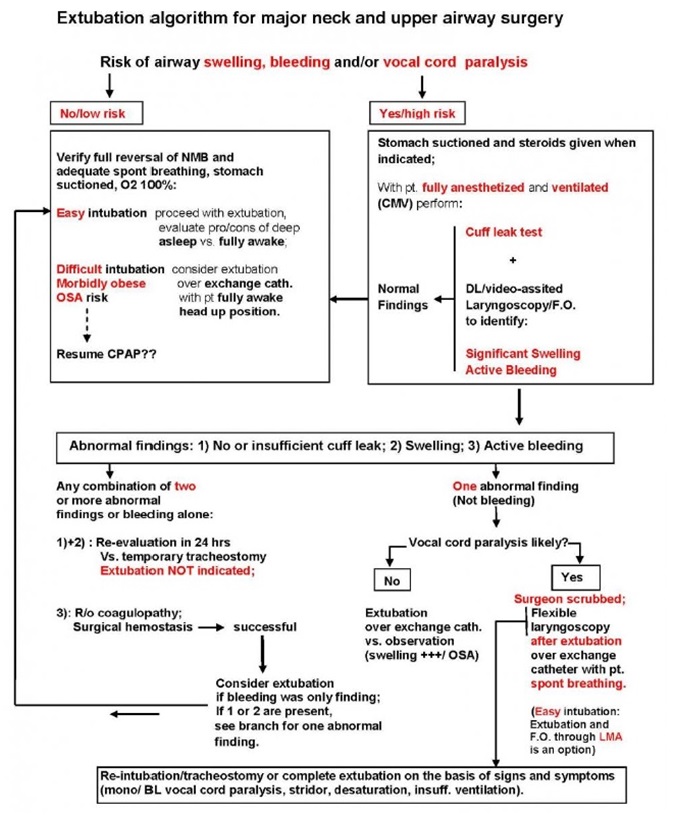
- Extubation Algorithm after Thyroidectomy Surgery
- C-Section Made Safer With Automated BP System:
| Introduction : | |||||||||||||||||
| People with a low platelet count (thrombocytopenia) often require lumbar punctures or an epidural anaesthetic. Lumbar punctures can be diagnostic (haematological malignancies, epidural haematoma, meningitis) or therapeutic (spinal anaesthetic, administration of chemotherapy). Epidural catheters are placed for administration of epidural anaesthetic. Current practice in many countries is to correct thrombocytopenia with platelet transfusions prior to lumbar punctures and epidural anaesthesia, in order to mitigate the risk of serious procedure-related bleeding. However, the platelet count threshold recommended prior to these procedures varies significantly from country to country. This indicates significant uncertainty among clinicians of the correct management of these patients. The risk of bleeding appears to be low but if bleeding occurs it can be very serious (spinal haematoma). Therefore, people may be exposed to the risks of a platelet transfusion without any obvious clinical benefit. | |||||||||||||||||
| In a recently published systemic review[1] in The Cochrane Library on "Use of platelet transfusions prior to lumbar punctures or epidural anaesthesia for the prevention of complications in people with thrombocytopenia" extensively analysed the effects of different platelet transfusion thresholds prior to a lumbar puncture or epidural anaesthesia in people with thrombocytopenia. | |||||||||||||||||
 |
|||||||||||||||||
| Thrombocytopenia | |||||||||||||||||
| Thrombocytopenia is defined as a platelet count less than 150 x 109/L (BCSH 2003), and severe thrombocytopenia as a platelet count less than 50 x 109/L. A platelet count less than 150 x 109/L occurs commonly in pregnancy (7% to 12% of pregnancies), but severe thrombocytopenia (platelet count less than 50 x 109/L) is much more uncommon (0.05% to 1% of pregnancies). A platelet count less than 150 x 109/L is very common in people with chronic liver disease (up to 76%). | In the general population, the risk of a spinal haematoma is very low (1 in 200,000 epidural anaesthetic procedures during labour to 1 in 3600 epidural anaesthetic procedures in older women having knee surgery). Risk factors for major bleeding are multifactorial and include: increasing age (the procedure is more difficult in older people due to changes to the spine that occur with age), low platelet count, abnormal coagulation (including anticoagulant medication) and traumatic needle or catheter insertion. Performing an LP or administration of epidural anaesthesia is a relative contraindication in people with thrombocytopenia due to this perceived higher risk of complications. However, overall, there are no current reliable estimates of the risks of adverse effects such as spinal haematomas in people with thrombocytopenia. | ||||||||||||||||
| Preoperative platelet transfusions | |||||||||||||||||
| Current practice in many countries is to correct thrombocytopenia with platelet transfusions prior to an LP or epidural anaesthesia, in order to mitigate the risk of serious peri- or post-procedural bleeding. Up to 4% of all platelet components issued in the UK prior to a procedure are given to people with thrombocytopenia who need an LP. | |||||||||||||||||
| The safe platelet count threshold recommended prior to an LP or epidural anaesthesia varies significantly from country to country. | |||||||||||||||||
| For example, the platelet count threshold for LP in the US is 50 x 109/L; in the UK it is 50 x 109/L in adults (BCSH 2003), but 20 to 40 x 109/L in children (BCSH 2004); and in Germany it is 20 x 109/L unless it is an urgent procedure (e.g. diagnosing bacterial meningitis) when an LP should be performed irrespective of the platelet count. | |||||||||||||||||
| The platelet count threshold for epidural anaesthesia also varies. In Italy and the UK, a platelet count of at least 50 x 109/L is recommended (BCSH 2003), while in France a platelet count of at least 80 x 109/L is recommended. | |||||||||||||||||
| As there is currently no consensus on the standard platelet count threshold prior to an LP or epidural anaesthesia, the above systemic review compared the most commonly recommended platelet count threshold in national guidelines (50 x 109/L) against other recommended thresholds (10 x 109/L, 20 x 109/L, 30 x 109/L, 40 x 109/L, 80 x 109/L). | |||||||||||||||||
| Authors' conclusions | |||||||||||||||||
| This review fails to provide any evidence to guide practice. | |||||||||||||||||
| There is no evidence from RCTs to determine what is the correct platelet transfusion threshold prior to insertion of a lumbar puncture needle or epidural catheter. There are no ongoing registered RCTs assessing the effects of different platelet transfusion thresholds prior to the insertion of a lumbar puncture or epidural anaesthesia in people with thrombocytopenia. Any future RCT would need to be very large to detect a difference in the risk of bleeding. We would need to design a study with at least 47,030 participants to be able to detect an increase in the number of people who had major procedure-related bleeding from 1 in 1000 to 2 in 1000. | |||||||||||||||||
| Another retrospective cohort study | |||||||||||||||||
| A retrospective cohort study[2] using the Multicenter Perioperative Outcomes Group database to identify thrombocytopenic parturients who received a neuraxial technique and to estimate the risk of epidural hematoma. Patients were stratified by platelet count, and those requiring surgical decompression were identified. | |||||||||||||||||
| A total of 573 parturients with a platelet count less than 100,000 mm–3 who received a neuraxial technique across 14 institutions were identified in the Multicenter Perioperative Outcomes Group database, and a total of 1,524 parturients were identified after combining the data from the systematic review. No cases of epidural hematoma requiring surgical decompression were observed. The upper bound of the 95% CI for the risk of epidural hematoma for a platelet count of 0 to 49,000 mm–3 is 11%, for 50,000 to 69,000 mm–3 is 3%, and for 70,000 to 100,000 mm–3 is 0.2%. | |||||||||||||||||
| Relative risks related to neuraxial blocks in obstetric patients with Thrombocyopenia - Regional Anaesthesia UK Recommendations: | |||||||||||||||||
| |||||||||||||||||
| Conclusions | |||||||||||||||||
The number of thrombocytopenic parturients in the literature who received neuraxial techniques without complication has been significantly increased. | |||||||||||||||||
Dr Joost van Veen et al[3] after analysing 17 national and international guidelines from blood transfusion and anaesthetic societies suggested that 80 × 109/l is a safe count for placing/removing an epidural or spinal anaesthetic and 40 × 109/l is a safe count for LP for general population. This, however, is provided that: | |||||||||||||||||
1 The platelet count is stable. | |||||||||||||||||
2 There is no other acquired or congenital coagulopathy. | |||||||||||||||||
3 The platelet function is normal and the patient is not on an antiplatelet drug. | |||||||||||||||||
4 The patient is not on an anticoagulant. If the patient is on a low molecular weight heparin, 12 h should have elapsed from the last dose of a prophylactic dose or 24 h after a therapeutic dose before an epidural or spinal anaesthetic is placed. | |||||||||||||||||
It is possible that lower platelet counts may also be safe but there is insufficient published evidence to make recommendations for lower levels at this stage. For patients with platelet counts of 50–80 × 109/l requiring epidural or spinal anaesthesia and patients with a platelet count 20–40 × 109/l requiring a LP, an individual decision based on risks and benefits should be made. | |||||||||||||||||
| References : | |||||||||||||||||
1. Estcourt LJ, Ingram C, Doree C, Trivella M, Stanworth SJ. Use of platelet transfusions prior to lumbar punctures or epidural anaesthesia for the prevention of complications in people with thrombocytopenia. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No.: CD011980. DOI: 10.1002/14651858.CD011980.pub2. | |||||||||||||||||
2. Risk of Epidural Hematoma after Neuraxial Techniques in Thrombocytopenic Parturients: A Report from the Multicenter Perioperative Outcomes Group. Anesthesiology 6 2017, Vol.126, 1053-1063. | |||||||||||||||||
3. Van Veen, J. J., Nokes, T. J. and Makris, M. (2010), The risk of spinal haematoma following neuraxial anaesthesia or lumbar puncture in thrombocytopenic individuals. British Journal of Haematology, 148: 15–25. doi:10.1111/j.1365-2141.2009.07899.x | |||||||||||||||||
| C-Section Made Safer With Automated BP System: | |||||
| C-Section Made Safer With Automated BP System: DIVA (Double Intravenous Vasopressor Automated) | |||||
Doctors at KK Women’s and Children’s Hospital (KKH), Singapore have developed the world’s first fully-automated system to enhance the management of blood pressure in women undergoing caesarean section under spinal anaesthesia. | |||||
The novel Double Intravenous Vasopressor Automated (DIVA) System detects and responds rapidly to low blood pressure and/or slow heart rate in real-time by autoadministering a precise amount of the recommended medication to manage reduced blood pressure (vasopressor). The system uses an advanced decision algorithm to ensure enhanced patient safety. | |||||
 |
|||||
Enhanced management of low blood pressure during c-section under spinal anaesthesia | |||||
Peri-operative hypotension during spinal anaesthesia for caesarean section is common owing to the loss of sympathetic tone and the physiological predisposition of pregnant women to hypotension. The consequences of maternal hypotension are potentially severe, affecting both the mother and child. Maternal effects include nausea, vomiting and headache attributable to inadequate organ perfusion; fetal adverse effects may include fetal acidosis and poor neurological outcomes because of a lack of placental vascular autoregulation, which renders its perfusion pressure-dependent. While several strategies, both pharmacological and nonpharmacological, have been used to treat hypotension, the optimal treatment is still debatable. Non-pharmaco-logical methods alone are often not effective; hence, the use of vasopressors is often required. Recent data support the use of alpha-1 adrenergic agonists such as phenylephrine, although ephedrine (a mixed alpha- and beta-adrenergic agonist) may be useful in the event of concomitant hypotension with bradycardia. | |||||
Setting a new standard in speed and accuracy, the DIVA System is an automated dual-pump system which innovatively integrates continuous non-invasive blood pressure monitoring to ensure closed-loop vasopressor administration during the surgery. It continuously measures and records blood pressure and heart rate every second, allowing the extremely rapid detection of blood pressure changes and episodes of low blood pressure. When low blood pressure and/or slow heart rate are detected, the DIVA System automatically and promptly administers the precise amount of the appropriate vasopressor required to restore optimal blood pressure – making the process much more efficient than manual administration. | |||||
Study: DIVA System clinically effective in management of blood pressure | |||||
B. L. Sng et al conducted a randomised, controlled, double-blinded trial involving 213 healthy women who underwent elective caesarean delivery under spinal anaesthesia using 11 mg hyperbaric bupivacaine with 15 lg fentanyl and 100 lg morphine. The automated vasopressor group had better systolic pressure control, with 37/106 (34.9%) having any beat-to-beat systolic pressure reading < 80% of baseline compared with 63/107 (58.9%) in the control group (p < 0.001). There was no difference in the incidence of reactive hypertension, defined as systolic pressure > 120% of baseline, with 8/106 (7.5%) in the automated vasopressor group vs 14/107 (13.1%) in the control group, or total dose of vasopressors. The automated vasopressor group had lower median absolute performance error of 8.5% vs control of 9.8% (p = 0.013), and reduced incidence of nausea (1/106 (0.9%) vs 11/107 (10.3%), p = 0.005). | |||||
Results showed that the DIVA System is clinically more effective in maintaining blood pressure during caesarean section under spinal anaesthesia, as it is able to detect and normalise blood pressure fluctuations more quickly and effectively than traditional physician administered techniques using conventional blood pressure monitoring. | |||||
How the DIVA System works | |||||
 |
|||||
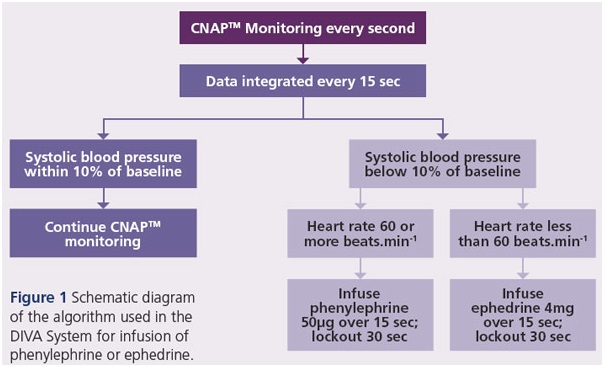
The DIVA System calculates the amount of vasopressor to administer using a customised algorithm based on integrated blood pressure data from a continuous non-invasive arterial blood pressure (CNAPTM) monitoring system. A closedloop feedback system controls vasopressor administration, ensuring that the patient receives only the precise dosage required (Figure 1). | |||||
 |
|||||
| |||||
| 2. The data is exported to a laptop computer, and integrated every fifteen seconds using a customised programme to determine the dosage of vasopressor required. | |||||
| 3.If low blood pressure occurs, phenylephrine is administered automatically via a syringe pump. If low blood pressure occurs in conjunction with slow heart rate, ephedrine is administered instead via a different syringe pump. | |||||
| 4. The vasopressor is administered over 15 seconds, followed by a 30-second lockout period to permit the vasopressor to take effect.> | |||||
Benefits of the DIVA System | |||||
| |||||
| |||||
| |||||
| |||||
Ref: | |||||
1. Sng BL, Wang H, Assam PN, Sia AT. Assessment of an updated double-vasopressor automated system using Nexfin for the maintenance of haemodynamic stability to improve peri-operative outcome during spinal anaesthesia for caesarean section. Anaesthesia. 2015 Jun;70(6):691-8. | |||||
2. Sing Health Medical News. https://www.singhealth.com.sg/Pages/home.aspx | |||||