| |
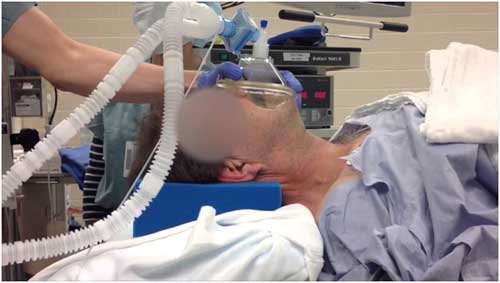
Should Anaesthesiologist to confirm effective mask ventilation before administering muscle relaxant? |
| |
Introduction: |
| |
ADEQUATE facemask ventilation (FMV) is the most fundamental and important skill for safe airway management during anesthesia induction as well as successful resuscitation. |
| |
It seems to be deeply embedded in the minds of most Anaesthesiologists that, following induction of anaesthesia, effective Face Mask Ventilation(FMV) must be established before any neuromuscular blocking drug (NMBD) is administered. |
| |
There is ongoing controversy as to whether effective facemask ventilation (FMV) should be established following induction of anesthesia before a muscle relaxant is administered. The rationale for such practice is the belief that, should FMV be ineffective, non-paralyzed patients can be woken up, and subsequently an alternative airway management can be considered. |
| |
Nevertheless, difficult or impossible FMV accompanied with difficult tracheal intubation during anesthesia induction occurs in 0.4% of adult anesthesia cases, possibly leading to life-threatening complications [1] |
| |
|
| |
Routine practice of induction of Anaesthesia |
| |
When the patient has become apnoeic following induction of anaestheia and if you are not able to ventilate with FMV the options available for the believers in “FMV should be established before administering muscle relaxants” are : |
| |
 |
Continue trying to establish FMV |
 |
They try to deepen anaesthesia by injecting additional dose of hypnotic agents (It will worsen hypoxia by promoting upper airway collapse and respiratory depression) |
 |
Insert an oro-pharyngeal airway to assist FMV |
 |
Awaken the patient. |
|
| |
In this situation of not able to ventilate the alternate options available are insertion of LMA or entotracheal intubation. However in the presence of complete lack of muscle relaxation such interventions are prone to fail. Most Anaesthetists are prepared to administer a NMBD when FMV remains impossible and the patient is at risk of becoming or already in hypoxia. |
| |
Muscle relaxation and quality of facemask ventilation |
| |
In the awake state the upper airway is maintained by physiological reflexes and neural activity of the airway muscles. In the unconscious state neuromuscular control of the upper airway is reduced or abolished. In addition immediately following induction of anaesthesia upper airway reflexes may increase which can lead to laryngospasm. Thus difficult or impossible FMV following induction of anaesthesia may be caused by soft tissue obstruction at the pharyngeal or laryngeal level and/ or by laryngospasm. The administration of NMBD can improve FMV by blunting or abolishing larngospasm, by reducing chest wall rigidity and by facilitating optimal sniffing position, jaw thrust and insertion of oro or nasopharyngeal airways. |
| |
What’s the Evidence? |
| |
Several studies have shown that administration of a NMBD following induction of anaesthesia facilitated FMV. |
| |
Warters et al [2] proved administration of rocuronium following induction of anaesthesia improved FMV in 28 of 42 patients (67%) without worsening any of them. |
| |
Sachdeva et al [3] showed During pressure-controlled FMV at 15 cmH2O of 125 anesthetized patients, following the administration ofvecuronium the expired tidal volume (a surrogate marker of ease of mask ventilation) significantly increased by a mean of 61 ml (12 %). Mean expired tidal volumes also increased significantly in patients with a body mass index (BMI) >30 kg/m`. A decrease was not observed in any of the patients. At first glance. a mean increase in expired tidal volume of 61 nil may seem clinically irrelevant. However, as the average oxygen consumption in a healthy subject is —250 ml/min, an increase in minute volume of 61 ml at 12 breaths/min amounts to 732 ml/min, which is equivalent to three times the oxygen consumption. |
| |
Amathieu R et al [4] conducted a prospective evaluation of an algorithm for difficult airway management in a total of 12,225 patients. Patients with established indications for awake fiberoptic intubation limited mouth opening, severe fixed deformity of the cervical spine, history of impossible tracheal incubation) were excluded from the study. Participating patients were prospectively subdivided into those with <3 and those with >3 risk factors for difficult airway management (risk factors being men aged >50 years, obesity with BMI > kg/m2, sleep apnea syndrome, Mallampati classes III and IV. mouth opening or intergingivial distance <35 mm, thyroid to mentum distance <65 mm, severely ilmited jaw protrusion, neck circumference >40 cm in women and 45 cm in men). The algorithm required that patients with >3 risk factors for difficult airway management were to receive suxamethonium without prior assessment of quality of FMV right after induction of anesthesia. This approach is by itself remarkable because it clearly runs against the traditional view that especially those patients at increased risk for airway problems should not be paralyzed before effective FMV has been demonstrated. |
| |
Amongst the entire population of 12,225 patients, there were 188 patients with >3 risk factors for difficult airway management who (in accordance with the algorithm) received suxamethonium right after induction of anesthesia. The quality of subsequent FMV was of grade I (ventilation without need for an oral airway) and grade II (ventilation requiring oropharyngeal airway) in 175 of these patients (93 %), of grade III (difficult and variable ventilation requiring an oral airway and two providers; or an oral airway and one provider using pressure-controlled mechanical ventilation requiring 25 cmH20) in 12 patients (6.4 %), and of grade IV (ventilation inadequate with no end-tidal carbon dioxide measurement and no perceptible chest wall movement during attempts at positive pressure) in 1 patient (0.5 %). |
| |
In those 12,003 patients with <3 risk factors for difficult airway management, the algorithm required assessment of quality of FMV before administration of a NMBD. In accordance with the algorithm, patients with grade I or II difficulty of FMV received a non-depolarizing NMBD (n = 11,943); patients with grade III (n = 90) difficulty of FMV received suxamethonium without any attempt at first improving the quality of FMV by whatever means or waking up the patient. This, again, is remarkable in itself because by administering a NMBD in the presence of difficult FMV, the option to 'wake up the patient' is effectively abolished. No case of FMV difficulty grade IV was observed after induction of anesthesia before administration of a NMBD. Most relevant in the context of this discussion, in 56 of the 90 patients (62 %) with FMV difficulty grade III, the quality of FMV improved by one grade following administration of suxamethonium. Equally important, in none of the 12,003 patients did the quality of FMV worsen following administration of the NMBD. In the entire population of 12,225 patients, there were 17 episodes of severe hypoxemia [peripheral oxygen saturation (Sp02) of less than 80 % until the time of endotracheal intubation]. Only three of the 17 episodes of severe hypoxemia were attributable to difficult FMV. This, study implies early muscle relaxation, independent of the quality of FMV, will definitely establish optimal ,intubating conditions as quickly as possible. |
| |
Aya Ikeda et al [5] studied the effect of muscle relaxants on FMV, and found that In 31 subjects with normal upper airway anatomy and head and mandible position held fixed during pressure-controlled ventilation, facemask ventilation was not worsened by rocuronium, but was improved by succinylcholine, especially at the time of fasciculations. The increase in airflow after succinylcholine was primarily because of an increase in oral ventilation. |
| |
Figure 1 shows typical changes of oral and nasal airflows following intravenous injection of rocuronium (A ) and succinylcholine (B ). Note the abrupt increase of airflow, particularly through oral airway route during the process of succinylcholine-induced muscle paralysis. Rocuronium injection did not change either oral or nasal airflows even at loss of train-of-four responses (fig. 2A). In contrast, after succinylcholine injection, airflows through both airway routes initially decreased, and then abruptly and significantly increased particularly through oral airway route (fig. 2B). Thereafter, the airflows gradually decreased but remained increased even after complete paralysis. |
| |
Figure 1: Typical changes of oral and nasal airflows following intravenous injection of rocuronium (A ) and succinylcholine (B ) |
| |
click here for image |
| |
Figure 2 : summarize changes of ventilatory parameters after succinylcholine injection. Onset of sudden changes (either increase or decrease) of airflow after succinylcholine injection was 23 s on average and did not differ between oral and nasal airway routes. The pattern of tidal volume changes was biphasic for both airway routes, as illustrated in figure 2. Although the airflow initially decreased at both airway routes, nasal tidal volumes decreased significantly more than oral tidal volume, as evidenced by significant differences of both tidal volume ratios and absolute tidal volumes between the airway routes. The maximum tidal volumes were achieved earlier in the oral airway route (35 s) than the nasal airway route (50 s). Although the achieved maximum tidal volumes after succinylcholine injection did not differ between the airway routes, ratios of the maximum tidal volumes to those of control condition were significantly greater in the oral route than the nasal route. These changes indicate preferential effects of succinylcholine-induced fasciculation on the oral airway passage. |
| |
Figure 2 : Time course of mean changes of nasal and oral tidal volumes after succinylcholine injection. |
| |
click here for image |
| |
Figure 3, Endoscopic observation of the space at the isthmus of the fauces during facemask ventilation in the process of muscle paralysis with intravenous administration of succinylcholine. The images were obtained by an endoscope inserted through the mouth. Note significant dilation of the airway space during succinylcholine-induced pharyngeal fasciculation. The narrowed space abruptly and significantly dilated during oscillatory movements of the soft palate and the tongue base (pharyngeal fasciculation). The dilated airway space gradually narrowed, but remained dilated more compared with the original luminal size before succinylcholine administration. Although extent and duration of the airway dilation immediately after the pharyngeal fasciculation varied among the subjects, the space at the isthmus of the fauces dilated in all six patients. |
| |
Figure 3: Endoscopic observation of the space at the isthmus of the fauces during facemask ventilation in the process of muscle paralysis with intravenous administration of succinylcholine. |
| |
click here for image |
| |
Aya Ikeda et al concluded in most cases, anesthesiologists use one hand alone for holding a mask. The hand often closes the bite and oral airway route. Even with the anesthesia full facemask, the mask ventilation is performed predominantly or exclusively through the nasal airway route without an oral airway in place or active bite opening with the two hands. Our results suggest use of both airway routes for maximizing ventilation in patients receiving muscle relaxants under general anesthesia, preferably with using an oral airway and/or two hands specifically in obese patients and patients with severe obstructive sleep apnea. |
| |
Conclusion |
| |
The overall evidence suggests that following the administration of a NMBD, the quality of FMV either remains unchanged or improves, but never worsens, irrespective of the initial quality of FMV. No airway technique under general anaesthesia is guaranteed to work always, but in airway management NMBDs seem to be much more often the answer than the problem. An underdosing of the anesthetic induction drug with the aim of maintaining the option of waking up the patient in the event of difficult FMV, may by itself render FMV more difficult. Lack of effective muscle relaxation will considerably reduce the chance of successful endotracheal intubation which may be required when hypoxemia develops during failed FMV. In a way, the reluctance to provide early effective muscle relaxation may actually cause rather than prevent a 'can't intubate. can't ventilate' situation. Moreover, early relaxation may reduce me risk or hypoxemia aria pulmonary aspiration by shortening the time interval between anesthesia-induced apnea and intubation. |
| |
Source : |
| |
1. Hans-Joachim Priebe. Should anesthesiologists have to confirm effective facemask ventilation before administering the muscle relaxant? J Anesth. 2016 Feb;30(1):132-7. |
| |
2. Aya Ikeda, M.D.; Shiroh Isono, M.D.; Yumi Sato, M.D.; Hisanori Yogo, M.D.; Jiro Sato, M.D.; Teruhiko Ishikawa, M.D.; Takashi Nishino, M.D. Effects of Muscle Relaxants on Mask Ventilation in Anesthetized Persons with Normal Upper Airway Anatomy. Anesthesiology 9 2012, Vol.117, 487-493. |
| |
References : |
| |
1. Kheterpal S, Han R, Tremper KK, Shanks A, Tait AR, O'Reilly M, Ludwig TA: Incidence and predictors of difficult and impossible mask ventilation. ANESTHESIOLOGY 2006; 105:885–91. |
| |
2. Warters RD1, Szabo TA, Spinale FG, DeSantis SM, Reves JG. The effect of neuromuscular blockade on mask ventilation. Anaesthesia. 2011 Mar;66(3):163-7. |
| |
3. Sachdeva R1, Kannan TR, Mendonca C, Patteril M. Evaluation of changes in tidal volume during mask ventilation following administration of neuromuscular blocking drugs. Anaesthesia. 2014 Aug;69(8):826-31. |
| |
4. Amathieu R. Combes X. Abdi W, Housseini LE, Rezzoug A , Dinca A. Slavov V, Bloc S, Dhonneur G. An algorithm for difficult airway management, modified for modern optical devices (Airtraq laryngoscope; LMA CTrachni): a 2-year prospective validation in patients for elective abdominal, gynecologic and thyroid surgery. Anesthesiology. 2011:114:25-33. |
| |
5. Aya Ikeda, M.D.; Shiroh Isono, M.D.; Yumi Sato, M.D.; Hisanori Yogo, M.D.; Jiro Sato, M.D.; Teruhiko Ishikawa, M.D.; Takashi Nishino, M.D. Effects of Muscle Relaxants on Mask Ventilation in Anesthetized Persons with Normal Upper Airway Anatomy. Anesthesiology 9 2012, Vol.117, 487-493. |