Conference Lectures
Introduction
Vitreo-retinal surgery is commonly done under peribulbar anaesthesia supplemented with intravenous sedation. The most commonly used medications for monitored anaesthesia care (MAC) are midazolam and fentanyl. The most commonly reported adverse effects of midazolam are variability of patient response and respiratory complications1 .Combining midazolam with fentanyl for MAC increases the risk of hypoxaemia and apnoea2.The adverse respiratory profile of benzodiazepine and opioids, associated postoperative nausea and vomiting(PONV) along with the stress response to surgery( tachycardia and hypertension ) create the need for a sedative drug that can be used safely during MAC, with limited adverse effects.
Dexmedetomidine is a centrally acting alpha – 2 receptor agonist that can be titrated to the desired level of sedation without significant respiratory depression3-5. The drug produces sedative – hypnotic, analgesic and anxiolytic effects by an action on alpha 2 receptors in the locus ceruleus6. It has sympatholytic effect that can attenuate the stress response to surgery, mitigating tachycardia and hypertension7. It is the primary sedative drug for orthopaedic , ophthalmic , dental, plastic surgery and for various diagnostic procedures8.The most frequent adverse effects reported in the literature are bradycardia and hypotension. Bradycardia and hypotension are noticed mainly with loading dose of Dexmedetomidine which can be avoided by omitting the loading dose or limiting the loading dose to 0.4 micrograms/kg 9. Hence , w e u s e d t w o d i ff e r e n t l o a d i n g d o s e s o f Dexmedetomidine and compared the same with midazolam – fentanyl combination.
Aim of the Study
- To compare haemodynamics, level of sedation, effect on respiration and surgeon satisfaction between midazolam-fentanyl and Dexmedetomidine.
- To r e c o m m e n d a s a f e l o a d i n g d o s e o f Dexmedetomidine for vitreo-retinal surgery.
Methods
After the approval of the Institutional Ethics Committee, this study was conducted involving 60 patients. Informed consent was taken from all the patients. Patients were randomly allocated into 3 groups of 20 each.
Inclusion criteria
- Patients aged between 40-70 years
- ASA Grade I – III
- Vitrectomy under Local anaesthesia and intravenous sedation
Exclusion criteria
- Baseline heart rate less than 60 / min
- Age more than 70 years
- Severe left ventricular dysfunction (EF<30%)
- Hypovolemia with systolic blood pressure less than 90mmhg
- Mobitz type 2 and 3rd degree heart block
- Severe cardiac disease (valve stenosis/regurgitation).
- Chronic renal failure and hepatic impairment.
Pre anaesthetic checkup done, investigated and fasting status of 4 hours followed. Patients reported to operation theatre (OT) complex 1 hour prior to surgery. Baseline vitals (HR ,SBP, DBP, RR, SpO2 ) noted and premedication T.Ranitidine 150 mg and T.Ondansetron 4 mg given with sips of water. Patients shifted to OT and basic monitors (ECG, NIBP, SpO2) applied and vitals noted before starting intravenous sedation. IV access secured and DNS/NS started depending on patient blood sugars. Oxygen supplemented with nasal cannula.
Group A (Midazolam – Fentanyl) patients received bolus IV midazolam 0.5 – 2mg (0.02 mg /kg) + fentanyl 12.5 – 25 micrograms. .
Group B patients received Dexmedetomidine 0.5 micrograms /kg IV loading dose over 10 mins on infusion pump (dilution 5microg/ml).
Group C patients received Dexmedetomidine 0.25 micrograms/kg loading dose over 10 mins on infusion pump (dilution 5microg/ml).
Peribulbar block given after 10 mins of starting sedation and surgery started after achieving adequate block. Vital parameters (HR,SBP, DBP, RR, SpO2) and level of sedation (LOS – RAMSAY SEDATION SCALE)10 noted every 5 mins for 1st 15 mins (5,10,15 ) and then every 15 mins till the end of surgery and then every 30 minutes for 2 hrs in the post operative ward. Level 3 Sedation targeted in Intraoperative period and achieved:
In Group A with aliquots of 0.5 – 1 mg of midazolam and 6.25-25 micrograms of fentanyl (11)as determined by level of sedation every 15 -30 mins.
In Group B and C with Dexmedetomidine titrated maintenance dose of 0.25 microg/kg/hr on infusion pump (titrations done with 0.1micg/kg/hr).
Adverse effects (bradycardia, hypotension, respiratory depression, level 4 sedation) noted and treated. 10 mins prior to the end of surgery, Dexmedetomidine infusion stopped and no additional bolus dose of sedatives given to group A patients. After completion of surgery, surgeon assessed for surgical comfort by rating the ease of performing surgery as -- Excellent (score3), Good (2), Fair (1), Poor (0). In the postoperative period, vital parameters noted every 30 min for 2 hours. Adverse effects like nausea and vomiting noted and treated with I.V Inj.Ondansetron 4mg. Post-operative pain treated with Inj Diclofenac 75mg i.m / Inj. Paracetamol 100ml I.V infusion. Patients were discharged when the ALDRETE SCORE (12) was >/= 9.
Statistical Analysis
Normality test, Kolmogorov-Smirnov and Shapiro Wilks tests results showed that the variables age, weight, duration of surgery, HR, SBP, DBP and RPP values follow normal distribution. Parametric tests are applied to analyse the data. One way ANOVA is used to compare the mean values between groups (If P value is < 0.05 then it is considered as statistically significant.)
Results
The demographic data of the 3 groups were comparable and no statistically significant difference noticed.
Haemodynamics
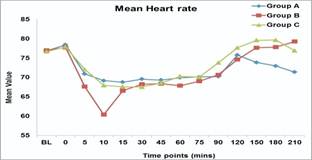
- Heart rate (HR): Heart rate values in group B were significantly lower than group A and C at 10 min. (P-value 0.001). Group A patients had lower HR than group B and C patients in the post-operative period (150, 180, 210).
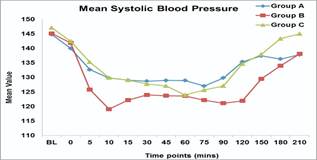
b) Systolic blood pressure (SBP): Group B patients had significantly lower SBP values at 10mins (P value-0.018), 90,120, 150,180, 210 (P value – 0.001) as compared to patients in group A and C. Lowest SBP in group B was noticed at 10 mins
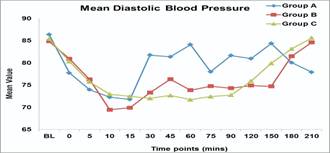
c) Diastolic blood pressure (DBP): Group B and C patients persistently had lower DBP than group A at various intervals 30, 45,60,75,90 , 120,150 min ( P value -0.001).
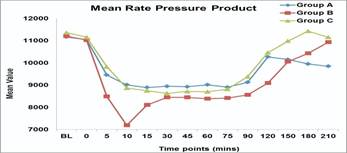
d) Rate pressure product (RPP): Group B patients had significantly lower RPP as compared to group A and C in the intra-operative period at 5, 10, 90 and 120 min (p value 0.001). Lowest RPP in group B was seen at 10 min.
2. Level of Sedation
Group B - Increased incidence of level 4 sedation predominantly in the 1st 30 min of I.V sedation.
3. Respiratory Parameters
a) Respiratory rate (RR): Clinically, group A and B patients had lower RR in the 1st 10 min of sedation as compared to group C; however the difference is not statistically significant and did not warrant any intervention.
b) Oxygen saturation: Clinically , oxygen saturation was lower in group B patients at 5 and 10 min intervals as compared to groups A and C; however patients needed no intervention and SpO2 increased once loading dose was completed and maintenance infusion dose started
4. Surgeon Satisfaction
Operating conditions were excellent in only 2 pts in group B as compared 7 in group A and 9 in group C. Poor operating conditions reported in 2 patients in group B and none in group A and C. Group B patients had statistically significant bradycardia ( P value < 0.001), hypotension ( p value -0.008) and level 4 sedation ( P value -0.001).Group A patients had significantly higher incidence of nausea ( P value 0.001) and vomiting ( P value - 0.002). Significant respiratory depression ( RR < 8/min or SpO2 < 90%) not seen in any of the groups.
Discussion
1. Cardiovascular Effects:
VENN et al14 showed that Dexmedetomidine at an initial loading dose of 1 micg/kg over 10 min followed by maintenance dose of 0.7micg/kg/hr resulted in adverse haemodynamic effects of either hypotension or bradycardia significantly during loading dose infusion.
BLOOR et al15 and TOBIAS et al16 in their study concluded that the potential adverse haemodynamic effects of Dexmedetomidine like bradycardia and hypotension occur with initial loading dose.
PETROZ et al17 in a study showed that the magnitude of decrease in heart rate and blood pressure was proportional to the dose of Dexmedetomidine and at lower doses , the decrease were of modest clinical interest and did not warrant corrective action.
In our study, there was significantly increased incidence of bradycardia and hypotension with Dexmedetomidine loading dose of 0.5micg/kg . However, such adverse effects were not significant when Dexmedetomidine loading dose was reduced to 0.25micg/kg.
2. Level of Sedation:
Keith A Candiotti et al13 in a study “ monitored anaesthesia care with Dexmedetomidine” indicated significantly increased ease of achieving and maintaining targeted sedation in Dexmedetomidine group when compared to midazolam.
Devangi A.Parikh et al18 compared Dexmedetomidine (1micg/kg loading dose - 0.2micg/kg/hr) with midazolam (0.06mg/kg) plus fentanyl (1micg/kg) for tympanoplasty and concluded that Dexmedetomidine is comparable to midazolam-fentanyl sedation.
In our study, group A patients needed repeated incremental doses to maintain target level of sedation; group B patients had higher incidence of level 4 sedation requiring frequent titration of maintenance infusion dose. Group C achieved and maintained target level of sedation easily.
3. Respiratory depression:
Bailey PL et al2 in his study , MAC with midazolam and fentanyl concluded that frequent hypoxaemia and apnoea are the complications after sedation with midazolam and fentanyl.
“Monitored Anaesthesia Care with Dexmedetomidine”- A study conducted by Keith A Candiotti et al13 concluded that the incidence of clinically significant respiratory depression (RR<8/min or SpO2 <90%) was lower in Dexmedetomidine treated patients as compared to patients treated with midazolam-fentanyl.
In our study, though the RR was lower in midazolam-fentanyl group ,it was not statistically significant and hypoxaemia requiring intervention was not noticed probably because of the lower dose of midazolam and fentanyl used. Significant respiratory depression not noticed in Dexmedetomidine group.
4. Nausea and Vomiting:
Holger K. Eltzschig et al19 and H.G. Mendel et al 20 conducted a study on children undergoing strabismus surgery and showed that use of opioids like fentanyl or remifentanyl significantly increased post-operative vomiting.
Our study observed same results. Group A patients had higher incidence of PONV (results in increased intraocular pressure), which can be detrimental in ophthalmic surgery.
5. Surgeon satisfaction:
“A prospective randomized double blind study comparing Dexemedetomidine vs combination of midazolam-fentanyl combination for tympanoplasty surgery under monitored anaesthesia care by Devangi A. Parilk18 showed better surgeon satisfaction with Dexedetomidine that Midazolam –fentanyl combination for sedation in tympanoplasty. In our study, surgeon satisfaction was better with lower loading dose of Dexmedetomidine (0.25micg/kg) when compared to 0.5micg/kg loading dose
The need for decreased loading dose of Dexmedetomidine especially in vitreo-retinal surgery may be explained by:
- Age group involved (mostly elderly)
- mostly diabetic patients (impaired autonomic nervous system)
- Procedure being done under peribulbar block( analgesia present)
- Level 4 sedation associated with sudden brisk response can result in ocular injury.
Conclusion
- Dexmedetomidine (lower loading dose) is a comparable, safe and effective primary sedative alternative to traditional midazolam – fentanyl combination for vitreo-retinal surgery under peribulbar anaesthesia.
- It can be a preferred mode of sedation for better control of intraoperative hypertension.
- Dexmedetomidine at loading dose of 0.25 µg/kg over 10 min followed by titrated maintenance dose of 0.25micg/kg/hr provides adequate level of sedation, stable haemodynamics and good surgeon satisfaction.
References
- ASA Task Force on Sedation and Analgesia by Non Anaesthesiologists. Practice guidelines for Sedation and Analgesia by Non Anaesthesiologists. Anesthesiology 2002 ;
- 96:1004-17.
- Bailey PL, Pace NL, Ashburn MA. Frequent Hypoxaemia and Apnoe after sedation with Midazolam and Fentanyl. Anaesthesiology 1990; 73: 826-30.
- Hall JE, Uhrich TD, Barney JA. Sedative, amnestic and analgesic properties of small dose Dexmedetomidine infusions. Anesth Analg 2000; 90:699-705.
- Ebert TJ, Hall JE, Barney JA. The effects of increasing plasma concentrations of Dexmedetomidine in humans. Anaesthesiology 2000; 93:382-94.
- Venn RM, Hell J, Grounds RM. Respiratory effects of Dexmedetomidine in the surgical patient requiring intensive care. Crit Care 2000; 4:302-8.
- Apan A, Doganci N, Ergan A. Bispectral index – guided intraoperative sedation with Dexmedetomidine and midazolam infusion in out patient cataract surgery. Minerva Anestesiol.
- 2009; 75:239-244.
- Abdalla MIM, Mansouri FA, Bener A. Dexmedetomidine during local anaesthesia. J Anesth 2006; 20: 54-6.
- Alhashemi JA. Dexmedetomidine Vs Midazolam for monitored anaesthesia care during cataract surgery. Br J Anesth 2006; 96: 722-6.
- Prasad SR, Simha PP, Jagadeesh AM. Comparative study between Dexmedetomidine and Fentanyl for sedation during mechanical ventilation in post operative paediatric cardiac surgical patients. Indian J Anesth 2012; 56: 547-52.
- Ramsay MA, Savege TM, Simpson BR. Controlled sedation with alphalaxone – alphadolone. BR Med J 1974; 2:656-9.
- Gross JB et all. Practice guidelines for sedation and analgesia. Anaesthesilogy 2002; 96: 1004-1017.
- Aldrete JA. The post anaesthesia recovery score revisited. J Clin Anesth 1995; 7: 89-91.
- Keith A Candiotti, Sergio D. Bergese, Paula M. Bokesch. Monitored Anaesthesia Care with Dexmedetomidine: A prospective, Randomized, Double – Blind, Multicenter Trial. Anesth Analg 2010; 110:47-56.
- Venn RM, Bradshaw CJ,Spencer R et all. Priliminary UK experience of Dexmedetomidine, a novel agent for post operative sedation in the intensive care unit. Anaesthesia 1999;
- 54: 1136-42.
- Bloor BC, Ward DS, Belleville JP. Effects of intravenous Dexmedetomidine in humans. II. Hemodynamic changes. Anaesthesilogy 1992; 77:1134-42.
- Tobias JD, Dexmedetomidine: applications in paediatric critical care and paediatric anesthesiology. Paediatr Crit Care Med 2007; 8: 115-31.
- Petroz GC, Sikich N, James M et al. A Phase I, two –center study of the pharmacokinetics and pharmacodynamics of Dexmedetomidine in children. Anesthesiology 2006 ; 105: 1098
- Devangi A Parikh, Sagar N Kolli. “A prospective randomized double blind study comparing Dexmedetomidine vs. combination of midazolam-fentanyl for tympanoplasty surgery under monitored anesthesia care. J Anaesthesiol Clin Pharmacol
- Holger K. Eltzschig, Torsten H. Schroeder, The effect of ramifentanyl or fentanyl on Post operative vomiting and pain in children undergoing Strabismus surgery. Aneth Analg 2002
- H.G.Mendel, K.M. Guarneiri. LM Sundtt and M.C. Torjman. The effects of ketorolac and fentanyl on post operative vomiting and analgesic requirements in children undergoing strabismus surgery. Anesth Analg 1995; 80: 1129-1133