Conference Lectures
Viscoelastic guidance of blood component therapy- a brief review.
Author Divya Amol Chandran Mahaldar MBBS, MD, DM
Affiliations: Consultant Cardiac Anaesthesiologist And Intensivist.
Manipal Hospital Goa, Dr E Borges Road, Dona Paula, Goa
1.0 Overview
- Thrombelastography (TEG®) was first described by hartert in 1948 as a method to assess the global hemostatic function from a single blood sample. 1
- Thromboelastography is a viscoelastic hemostatic assay that measures the global visco-elastic properties of whole blood clot formation under low shear stress
- It shows the interaction of platelets with the coagulation cascade (aggregation, clot strengthening, fibrin cross linking and fibrinolysis)
- Does not necessarily correlate with blood tests such as INR, APTT and platelet count (which are often poorer predictors of bleeding and thrombosis)
- This article briefly reviews TEG® and ROTEM® an alternative viscoelastic hemostatic assay that is widely available
2.0 Method
2.1 TEG
- TEG® measures the clot’s physical property by using a stationary cylindrical cup that holds the blood sample (heated to 37°C) and oscillates through an angle of 4°45′.
- Each rotation cycle lasts 10 s. A pin is suspended in the blood by a torsion wire and is monitored for motion. The torque of the rotation cup is transmitted to the immersed pin only after fibrin-platelet bonding has linked the cup and pin together.
- The strength of these fibrin-platelet bonds affects the magnitude of the pin motion.
- Thus, the output is directly related to the strength of the formed clot.
- As the clot retracts or lyses, these bonds are broken and the transfer of cup motion is again diminished.
- The rotation movement of the pin is converted by a mechanical-electrical transducer to an electrical signal, finally being displayed as the typical TEG® tracing. The tracing is shown in figure 1
- Point of care test (quick, takes around 30min)
- Rapidteg®-kaolin and kaolin + tissue factor (tf) are used as activators,
- NATEM -TEG® using native whole blood is slower
- Other tests are available including functional fibrinogen, a measure of fibrin-based clot function, and multiplate which evaluates platelet function
2.2. ROTEM®
- This uses a modified technology:
- The signal of the pin suspended in the blood sample is transmitted via an optical detector system, not a torsion wire
- The movement is initiated from the pin, not the cup
- Furthermore, the instrument is equipped with an electronic pipette
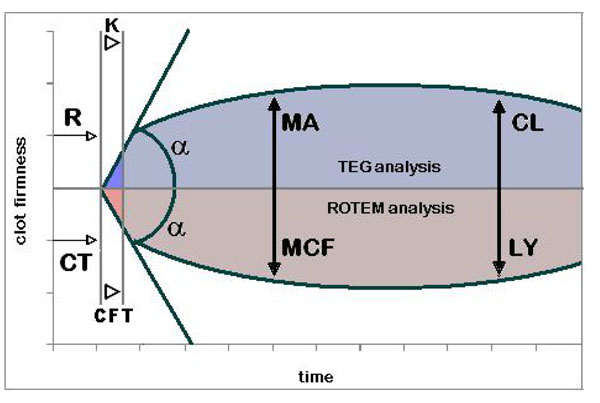
- TEG® and ROTEM® tracing TEG® parameters: R – reaction time; k – kinetics;
![[proportional, variant]](con-0dummy_clip_image002_0001.gif) - alpha angle; MA – maximum amplitude; CL – clot lysis. ROTEM® parameters: CT – clotting time; CFT – clot formation time;
- alpha angle; MA – maximum amplitude; CL – clot lysis. ROTEM® parameters: CT – clotting time; CFT – clot formation time; ![[proportional, variant]](con-0dummy_clip_image002_0002.gif) - alpha angle; MCF – maximum clot firmness; LY – clot lysis.
- alpha angle; MCF – maximum clot firmness; LY – clot lysis.
2.3 Specific Parameters Measured by viscoelastography represent the 3 phases of the cell-based model of haemostasis:
Initiation, Amplification, and Propagation
- Initiation -R value = reaction time (s); time of latency from start of test to initial fibrin formation (amplitude of 2mm)
- Amplification -k = kinetics (s); time taken to achieve a certain level of clot strength (amplitude of 20mm)
- Rate of clot formation -alpha angle (slope between r and k); measures the speed at which fibrin build up and cross linking takes place
- Time to maximum amplitude- TMA(s)
- Overall stability of the clot -MA -maximum amplitude (mm); represents the ultimate strength of the fibrin clot
- Degree of fibrinolysis -A30 or LY30 = amplitude at 30 minutes; percentage decrease in amplitude at 30 minutes post-ma and gives measure
- CLT = clot lysis time (s)
These values however are not identical in both the tests and interpreted as shown in table 1
3.0 Use
The primary application of these tests is to predict the need for transfusion and guide transfusion strategy.
Studies show cost-effectiveness and reduction in blood products in:
- Liver transplantation
- Cardiac surgery
May be useful in:
- Trauma (reduction in blood product use and mortality in cohort studies)
- Obstetrics (some data to show that it may decrease transfusion rates; this is controversial)
- Early detection of dilutional coagulopathy
The following situations might interfere in the interpretation of the results
- LMWH
- Aspirin
- Post cardiac bypass
- Fibrinolysis
- Hypercoagulability
Limitations of this technology
- Requires calibration 2-3 times daily
- Should be performed by trained personnel
- Susceptible to technical variations
3.1 TEG as a guide to treatment
- Increased r time => FFP
- Decreased angle => cryopreciptate
- Decreased ma => platelets (consider ddavp)
- Fibrinolysis => tranexamic acid (or aprotinin or aminocaproic acid)
3.2 TEG® versus ROTEM®
Compared in table 1
Test |
|
|
Method |
|
|
|
|
|
Measured variables |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.3 Comparison with plasma clotting tests
Pros of viscoelastic hemostatic assays
- Assessment of global haemostatic potential provides more information than time to fibrin formation
- Can readily differentiate a coagulopathy due to low fibrinogen from one due to thrombocytopenia
- Point-of-care (poc) device with rapid turnaround times so that many results available within 5–10 min of starting the test
Cons of viscoelastic hemostatic assays
- Variable availability
- Marked inter-operator variability and poor precision
- May require specialist staff to perform
4.0 Bibliography
1. Afshari A, Wikkelsø A, Brok J, Møller Am, Wetterslev J. Thrombelastography (Teg) Or Thromboelastometry (ROTEM) To Monitor Haemotherapy Versus Usual Care In Patients With Massive Transfusion. Cochrane Database Syst Rev. 2011 Mar 16 ;(3):Cd007871
2. Bolliger D, Seeberger Md, Tanaka Ka. Principles And Practice Of Thromboelastography In Clinical Coagulation Management And Transfusion Practice. Transfus Med Rev. 2012 Jan; 26(1):1-13
3. Da Luz Lt, Nascimento B, Rizoli S. Thrombelastography (Teg(R)): Practical Considerations On Its Clinical Use In Trauma Resuscitation. Scand J Trauma Resusc Emerg Med. 2013 Apr 16; 21(1):29.
4. Ganter Mt, Hofer Ck. Coagulation Monitoring: Current Techniques And Clinical Use Of Viscoelastic Point-Of-Care Coagulation Devices. Anesth Analg. 2008 May;106 (5):1366-75.