Conference Lectures
TRANSCRANIAL DOPPLER AND
NEAR INFRARED SPECTROSCOPY
Prof. H.H. Dash and Virendra Jain
Deptt.ofAnaesthesiology and Pain Medicine, Fortis Memorial Research Institute, Gurgaon
HISTORY
Aaslid and colleagues first reported the ability to record blood flow velocity in the intracranial arteries using Doppler ultrasound in 1982, introducing TCD ultrasonography.[1] TCD ultrasonography employed an optimized 2 MHz frequency with a pulsed Doppler range gated design. The lower 2 MHz frequency allowed penetration through the cranium in the thin portion of the bone.
The introduction of TCD ultrasound allowed examination of the intracranial vasculature, which has improved the diagnosis of intracranial disease. TCD ultrasound initially was employed in the department of Neurosurgery in Bern, Switzerland, for the diagnosis of vasopressin after subarachnoid hemorrhage.[2]
TRANSCRANIAL DOPPLER
Trans cranial Doppler ultrasonography has gained wide popularity in the very early phase, as well during the repetitive assessment of patients with cerebrovascular diseases owing to its non-invasiveness and easy applicability. Over the past decade, Power M mode, color Doppler imaging, and use of ultrasound contrast agents have extended the scope of TCD clinical applications. TCD is based on the principle that the cerebral blood flow velocity (CBFV) in a given artery is inversely related to the cross-sectional area of that artery. Thus, TCD provides an indirect evaluation of the vessel diameter by calculating the Doppler shift. [3]
Specific clinical applications of TCD includes diagnosing and monitoring vasospasm in patients with SAH of different aetiologies (aneurysm rupture, TBI and cerebral tumour resection) along with the assessment of various therapeutic modalities, such as stellate ganglion block, on cerebral haemodynamics [Fig. 1,2] . [4-6] TCD can also be used to evaluate other neurologic conditions in the Neurosciences Critical Care. Unit such as intra- and extracranial vascular stenosis, acute Ischaemic stroke (AIS), arteriovenous malformations, venous sinus thrombosis and intraoperative emboli.
Figure 1.Transcranial Doppler flow velocity in Right Middle Cerebral Artery in a patient with cerebral vasospasm before stellate ganglion block:
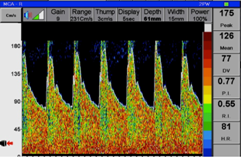
Figure 2.Transcranial Doppler flow velocity in Right Middle Cerebral Artery in the same patient with cerebral vasospasm 30 minutes after Stellate Ganglion Block:
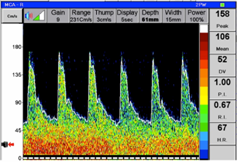
The sensitivity and specificity of TCD in predicting vasospasm vary according to the vessel, diagnostic criteria, and timing of correlative angiography. [7] In addition, CBFV’s on TCD can be influenced by technical issues (absence of temporal bone windows), vessel anatomy, and skills of operator. Moreover, episodic vasospasm may be missed because of intermittent measurements. [8]TCD appears to be highly predictive of an angiographically demonstrated vasospasm in the middle cerebral artery (MCA); however, its diagnostic accuracy is lower with regard to vasospasm in the basilar artery and anterior cerebral artery (ACA).
Lindegaard Index (LI) is defined as the ratio of the mean CBFV of the MCA to that of extra cranial portion of the ipsilateral extra cranial (proximal) internal carotid artery (ICA). This ratio increases with the severity of vasospasm. Normal values for this index ranges from 1.1 to 2.3 (median 1.7 at days 1–2) and in the absence of vasospasm is less than 3. If the CBFV is found to be elevated but the ratio is less than 3, then the elevation is thought to be due to hyperemia. LI is of value in distinguishing cerebral vasospasm from global hyperemia, especially in the setting of Triple H therapy in patients with aneurysmal SAH. [9]
TCD can be used to evaluate intracranial steno-occlusive disease, particularly in the terminal ICA, ICA siphon, and MCA. The American Academy of Neurology (AAN) Report of the Therapeutics and Technology Assessment Subcommittee mentions that TCD can detect acute MCA occlusions with greater than 90% sensitivity and specificity. [10] Other variables measured with TCD waveform are a Pulstality Index (PI) or Gosling Index and Resistance Index or Pourcelot Index; both reflecting the amount of resistance in the more distal cerebral blood vessels. [3]
PI = (PSV- EDV)/ Mean CBFV and RI = (PSV – EDV)/PSV.
[PSV: Peak Systolic CBFV; EDV: End-Diastolic CBFV]
Pulstality Index has been evaluated as an alternative to direct ICP measurement. Bellneret al. found that the ICP value predicted from the PI was within ± 4.2 mm Hg of the actual ICP, with a 95% confidence interval, in the ICP range of 5 to 40 mm Hg. [11]
In normal individuals, MCA CBFV changes by approximately 2.5%-3% for every mmHg change in PaCO2. TCD can therefore be used to assess the cerebrovascular reserve and cerebral vasoreactivity to carbon-dioxide in many clinical situations. [12]TCD may also be used to monitor the integrity of pressure auto regulation and can provide an insight into both rapid and delayed components of cerebral auto regulatory mechanisms.
TCD screening have been recommended as a practice standard in early ischemic stroke revealing dynamic changes in cerebral circulation that may be missed on a single neuroimaging study. [13]
Fast insonation protocols have been developed for rapid diagnosis and early thrombolytic therapy in AIS. [14] After thrombolysis, TCD facilitates continuous monitoring for detection of re-occlusion, distal occlusion, restenosis and recanalization and also to identify hyperemia. In its only therapeutic role, TCD per se facilitated breaking down the thrombus and assessment of recanalization during tissue plasminogen activator (t-PA) therapy in the CLOTBUST II trial for AIS [15] The Stroke Prevention Trial in Sickle Cell Anemia (STOP Trial) provided level IA evidence for use of TCD as a guide to help decide timing and frequency of transfusion therapy as a means to reduce the risk of a first stroke by 90% in this population.[16]
Contrast TCD performed with Echovist-300 (D-galactosemicroparticulate) has been found to yield 100% sensitivity to identify TEE-proven cardiac right-to-left shunts. [17] Power M-mode / TCD facilitates the location of the acoustic temporal windows and allows viewing blood flow from multiple vessels at the same time. Trans cranial color-coded duplex sonography (TCCS) allows 2-dimensional representation of the large cerebral arteries in colour with outlining of parenchymal structures, in addition to color-coded flow directionality information. Compared with TCD, TCCS allowed for the detection of vasospasm at an earlier stage and at lower velocities (using a threshold of 120 cm/s), which may allow for more timely interventions to arrest the complications of vasospasm when it occurs.[18]
TCD provides information on the flow velocity, direction of flow, shape of the Doppler waveform, and also differences in pulsatility amplitudes between systolic and diastolic CBFV, which can be used to support diagnosis of brain death. [19] The AAN Practice Parameters for Determining Brain Death in Adults considers TCD a confirmatory test of brain death along with clinical testing and other allied tests.
NEAR-INFRARED SPECTROSCOPY
Near-infrared spectroscopy (NIRS) is a noninvasive means of determining real-time changes in regional oxygen saturation (rSO2) of cerebral and somatic tissues.NIRS devices rely on the Beer-Lambert law, which states that one can measure a concentration of a substance based on its absorption of light.The absorption is proportional to the concentration of certain chromophores, mainly iron in haemoglobin and copper in cytochrome aa3. In the brain, the primary light-absorbing molecules within the near-infrared range are metal complex chromophores: oxyhaemoglobin, deoxyhaemoglobin, and cytochrome c oxidase (CCO).
Because most hemoglobin in tissue is in the venous circulation, NIRS gives a venous-weighted relative oxygen index of tissue beneath the probe. In adults, bilateral frontal cerebral oximetry is used to monitor perfusion to at risk areas of grey matter within cerebral cortex in the watershed areas between the ACA and MCA [Fig. 3]. The smaller head circumference of neonates and children permits greater depth of penetration of and assessment of subcortical tissue oxygenation.
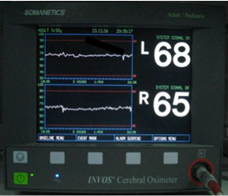
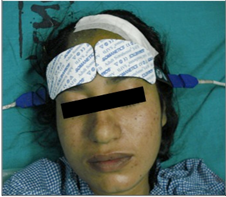
Figure 3.INVOS Cerebral Oximeter andBilateral Frontal Cerebral Oximetry
Recent developments in in newer spatially resolved NIRS with time- or frequency-resolved techniques or dual-path subtraction techniques have partially addressed the limiting issue of scattering of NIR light produced by superficial tissues beneath the sensor and reflectance of surviving light photons. [20]
NIRS-based techniques have real potential for identifying cerebral ischemia/hypoxia, characterize the cerebral hemodynamic and metabolic responses associated with neuronal function and dysfunction, and guide therapeutic brain protection strategies, particularly during carotid endarterectomy [21], cardiac surgery [22], major abdominal surgeries [23] and surgeries performed in steep head –up position. [24]
Measuring brain oxygenation might be a surrogate of the adequacy of non-neurologic organ perfusion. An rSO2 less than 40 – 50% or decline more than 20% of baseline is associated with hypoxic- ischemic neural injury. [25] In general, a decrease in rSO2 is reflective of an increase in oxygen extraction and debt as a result of increased metabolism, decreased perfusion, and/or stagnant perfusion. High rSO2 may be indicative of increased perfusion, decreased tissue bed metabolism, and/or less oxygen extraction.
In patients with SAH, episodes of angiographic vasospasms were associated with 24 ± 4% rSO2 reductions. [26] In TBI, an association has been described between rsO2 values <60% and mortality, intracranial hypertension, and compromised CPP. [27]
On comparison with SjvO2 and PbtO2 monitoring for measuring cerebral oxygenation, rSO2 had low accuracy for detecting moderate cerebral hypoxia (PbtO2 ≤ 15 mmHg) and was moderately accurate for detecting severe cerebral hypoxemia (PbtO2 ≤ 12 mmHg). [28] NIRS changes precede changes in ICP in patients having delayed traumatic hematomas [29]
Notably, cerebral oximetry is of limited value in TBI and SAH as cerebral edema or haemorrhage largely affects NIRS signals, and hence should be used as an additional neuromonitoring tool. However, this disadvantage has been put to use for identifying intracranial hematomas [30] and cerebral edema [31]. It has also been found useful to detect differences in cerebral haemodynamic responses of brain-injured patients to postural changes in the neurocritical care unit. [32]
NIRS also appears a useful technique to monitor adequate treatment for extracorporeal membrane oxygenation [33]; reflect adequacy of CPR measures after cardiopulmonary arrest [34]; track the quality of cerebral reperfusion and reoxygenation[35] and manage circulation in the newborn [36].NIRS in premature infants has been used to identify hypoxic and hyperemic states associated with adverse neurologic outcomes.
NIRS has been used to test CBF auto regulation. [37] It has been employed to determine the lower threshold limit for auto regulation during both normothermia and hypothermia and thus, might help adjusting CPP in many cardiac arrest victims. [38] NIRS-derived measurement of CCO has been validated as a potential biomarker of cellular metabolic state in the clinical setting. [39]
Direct measurements of CBF using DCS have been found superior to NIRS surrogates. DCS monitoring has the potential to detect changes in cerebrovascular physiology while ischemic changes are still reversible. The new hybrid technology of DCS for measurement of CBF, and NIRS for measurement of oxy- and deoxyhaemoglobin concentration provides comprehensive assessment of cortical blood flow and oxygenation using a single device and the same probes. [40] A prototype invasive probe combining NIRS and indocyanine green dye dilution has also been used to simultaneously monitor ICP, CBF, and CBV. [41]
Despite its potential advantages over other neuromonitoring techniques such as being user-friendly, non- invasive, and measurements over multiple regions of interest simultaneously with high temporal resolution; further investigation and technological advances are necessary before it can be introduced more widely into clinical practice. Various potential uses of NIRS in critical care setting as a monitoring tool for cerebral edema in diabetic ketoacidosis, the neonatal acute abdomen, hydrocephalus and shunt malfunction, prehospital care of trauma patients, compartment syndrome, shock, dehydration, and sepsis are currently under active investigation.
References:
- Aaslid R, Markwalder T-M, Normes H: Non invasivetranscranial Doppler ultrasound recording of a flow velocity in basal cerebral arteries. J Neurosurg 1982; 57:769
- Aaslid R, Huber P, Normes H: Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg 1984; 60:37-41
- White H, Venkatesh B. Applications of transcranial Doppler in the ICU: a review. Intensive Care Med 2006;32:981–94
- Gupta MM, Bithal PK, Dash HH, Chaturvedi A, Mahajan RP. Effects of stellate ganglion block on cerebral haemodynamics as assessed by transcranial Doppler ultrasonography. Br J Anaesth. 2005;95:669-73
- Prabhakar H, Jain V, Rath GP, Bithal PK, Dash HH. Stellate ganglion block as alternative to intrathecalpapaverine in relieving vasospasm due to subarachnoid hemorrhage. AnesthAnalg. 2007;104:1311–2
- Jain V, Rath GP, Dash HH, Bithal PK, Chouhan RS, Suri A. Stellate ganglion block for treatment of cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage - A preliminary study. J AnaesthesiolClinPharmacol. 2011;27:516-21
- Washington CW, Zipfel GJ. Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit Care 2011;15:312–7
- Venkatesh B, Shen Q, Lipman J. Continuous measurement of cerebral blood flow velocity using transcranial Doppler reveals significant moment-to- moment variability of data in healthy volunteers and in patients with subarachnoid haemorrhage. Crit Care Med 2002;30(3):563–9
- Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. ActaNeurochirSuppl (Wien) 1988;42:81–4
- Sloan MA, Alexandrov AV, Tegeler CH, et al. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004;62:1468–81
- Bellner J, Romner B, Reinstrup P, et al. Transcranial Doppler sonographypulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004;62:45–51; discussion 51
- Sharma D, Bithal PK, Dash HH, Chouhan RS, Sookplung P, Vavilala MS. Cerebral autoregulation and CO2 reactivity before and after elective supratentorial tumor resection. J NeurosurgAnesthesiol. 2010;22:132-7
- Akopov S. Haemodynamic studies in early ischemic stroke: serial transcranial Doppler and magnetic resonance angiography evaluation. Stroke 2002; 33:1274–9
- Burgin WS, Malkoff M, Felberg RA, et al. Transcranial Doppler ultrasound criteria for recanalization after thrombolysis for middle cerebral artery stroke. Stroke 2000; 31:1128 –32
- Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 2004; 351:2170– 8
- Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108: 847–52
- Droste DW. Optimizing the technique of contrast transcranial Doppler ultrasound in the detection of right-to-left shunts. Stroke 2002;33:2211–6
- Swiat M, Weigele J, Hurst RW, et al. Middle cerebral artery vasospasm: transcranial color-coded duplex sonography versus conventional nonimagingtranscranial Doppler sonography. Crit Care Med 2009;37:963–8
- Kaneko Z: First steps in the development of the Doppler flow meter. Ultrasound Med Biol 12:1877-1895; 1986
- Ghosh A, Elwell C, Smith M. Review article: cerebral near-infrared spectroscopy in adults: a work in progress. AnesthAnalg. 2012;115:1373-83
- Pennekamp CW, Bots ML, Kappelle LJ, Moll FL, de Borst GJ. The value of near-infrared spectroscopy measured cerebral oximetry during carotid endarterectomy in perioperative stroke prevention: a review. Eur J VascEndovascSurg 2009;38:539–45
- Fedorow C, Grocott HP. Cerebral monitoring to optimize outcomes after cardiac surgery. CurrOpinAnaesthesiol 2010;23:89–94
- Casati A, Fanelli G, Pietropaoli P, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. AnesthAnalg. 2005;101:740-7
- Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. AnesthAnalg 2010;111:496–505
- Hoffman GM, Ghanayem NS, Tweddell JS. Noninvasive assessment of cardiac output. SeminThoracCardiovascSurgPediatr Card SurgAnnu. 2005:12-21
- Bhatia R, Hampton T, Malde S, et al. The application of near-infrared oximetry to cerebral monitoring during aneurysm embolization: a comparison with intraprocedural angiography. J NeurosurgAnesthesiol 2007;19:97 –104
- Dunham CM, Ransom KJ, Flowers LL, Siegal JD, Kohli CM. Cerebral hypoxia in severely brain-injured patients is associ- ated with admission Glasgow Coma Scale score, computed tomographic severity, cerebral perfusion pressure, and survival. J Trauma 2004;56:482–9
- Leal-Noval SR, Cayuela A, Arellano-Orden V, et al. Invasive and noninvasive assessment of cerebral oxygenation in patients with severe traumatic brain injury. Intensive Care Med 2010;36:1309–17
- Gopinath SP, Robertson CS, Contant CF, et al. Early detection of delayed traumatic intracranial hematomas using near-infrared spectroscopy. J Neuosurg 1995; 83:438 –44
- Robertson CS, Gopinath SP, Chance B. A new application for near-infrared spectroscopy: detection of delayed intracranial hematomas after head injury. J Neurotrauma 1995;12:591–600
- Gill AS, Rajneesh KF, Owen CM, Yeh J, Hsu M, Binder DK. Early optical detection of cerebral edema in vivo. J Neurosurg 2011;114:470–7
- Kim MN, Edlow BL, Durduran T, et al. Continuous Optical Monitoring of Cerebral Hemodynamics During Head-of-Bed Manipulation in Brain-Injured Adults. Neurocrit Care. 2014;20:443-53
- Wong JK, Smith TN, Pitcher HT, et al. Cerebral and lower limb near-infrared spectroscopy in adults on extracorporeal membrane oxygenation. Artif Organs 2012; 36:659 –67
- Parnia S, Nasir A, Shah C, Patel R, Mani A, Richman P. A feasibility study evaluating the role of cerebral oximetry in predicting return of spontaneous circulation in cardiac arrest. Resuscitation.2012;83:982-85
- Taccone FS, Fagnoul D, Rondelet B, et al. Cerebral oximetry during extracorporeal cardiopulmonary resuscitation. Crit Care 2013; 17:409
- Wong FY, Leung TS, Austin T, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Paediatrics. 2008;121:e604-e611
- Steiner LA, Pfister D, Strebel SP, et al. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care 2009;10:122 –8
- Lee JK, Brady KM, Mytar JO, et al. Cerebral blood flow and cerebrovascular autoregulation in a swine model of paediatric cardiac arrest and hypothermia. Crit Care Med. 2011;39:2337-45
- Tisdall MM, Tachtsidis I, Leung TS, Elwell CE, Smith M. Near- infrared spectroscopic quantification of changes in the concentration of oxidized cytochrome c oxidase in the healthy human brain during hypoxemia. J Biomed Opt 2007;12:024002
- Kim MN, Durduran T, Frangos S, et al. Noninvasive Measurement of Cerebral Blood Flow and Blood Oxygenation Using Near-Infrared and Diffuse Correlation Spectroscopies in Critically Brain-Injured Adults. Neurocrit Care. 2010;12:173–80
- Keller E, Froehlich J, Muroi C, et al. Neuromonitoring in intensive care: a new brain tissue probe for combined monitoring of intracranial pressure (ICP) cerebral blood flow (CBF) and oxygenation. ActaNeurochirSuppl 2011;110:217–20